Atoms are the building blocks of everything you see, touch, taste, and even breathe. Whether you’re looking at a granite mountain, a drop of water, or your own body, it’s all made of atoms. Despite their tiny size—so small you can’t see them even with a regular microscope—atoms determine the structure and behavior of all matter. But what exactly are atoms? Where do they come from? And why do they matter so much in chemistry?
My journey with atoms began long before I ever wore a lab coat.
As a young student in primary school, I often found myself staring at ordinary objects—stones, leaves, droplets of rain—wondering, What are they made of? What’s inside them? That curiosity stayed with me. In secondary school, I was introduced to atomic structure, and for the first time, I could name what I had been wondering about: atoms—tiny particles that make up everything.
At university, I dived deeper into chemistry, learning about electron configurations, chemical bonding, and even the strange world of quantum mechanics.
During my Master’s in Chemistry, I worked with analytical tools that detected and quantified substances, all of which, I now knew, were composed of atoms. The precision of chemical analysis only deepened my fascination: how could something so small—invisible even under a normal microscope—hold the key to understanding the world?
And so, the question still begs: what are atoms?
Let me take you through this topic—not just from a textbook, but from the perspective of someone who has studied atoms across all levels of science. Let’s explore the atom—clearly, simply, and completely.
Table of Contents
The Birth of the Atom Concept
The word “atom” comes from the Greek word atomos, meaning “uncuttable” or “indivisible.” Around 400 BCE, Greek philosopher Democritus proposed that matter is made up of small, indivisible particles. His ideas lacked experimental support, so they remained philosophical for centuries.
It wasn’t until the 1800s that atomic theory gained scientific footing. British chemist John Dalton revived the idea, suggesting that:
- All matter is made of tiny, indivisible particles called atoms.
- Atoms of the same element are identical.
- Atoms of different elements combine in fixed ratios to form compounds.
- Atoms are rearranged in chemical reactions but never created or destroyed.
Dalton’s atomic theory laid the groundwork for modern chemistry, though we now know atoms are not indivisible—they’re made of smaller particles.
What Is an Atom? Definition and Meaning
The definition of an atom is:
The smallest unit of an element that retains the chemical properties of that element.
For instance, a gold atom is the smallest piece of gold that still behaves like gold.
An atom is the smallest unit of an element that can take in a chemical reaction.
In simple words, an atom is a tiny particle that makes up everything around you. It’s like the basic building block of all matter—kind of like a Lego brick for the universe.
Chemists study atoms to understand the structure of substances, how materials interact, and how new substances form. This is the foundation of chemistry.
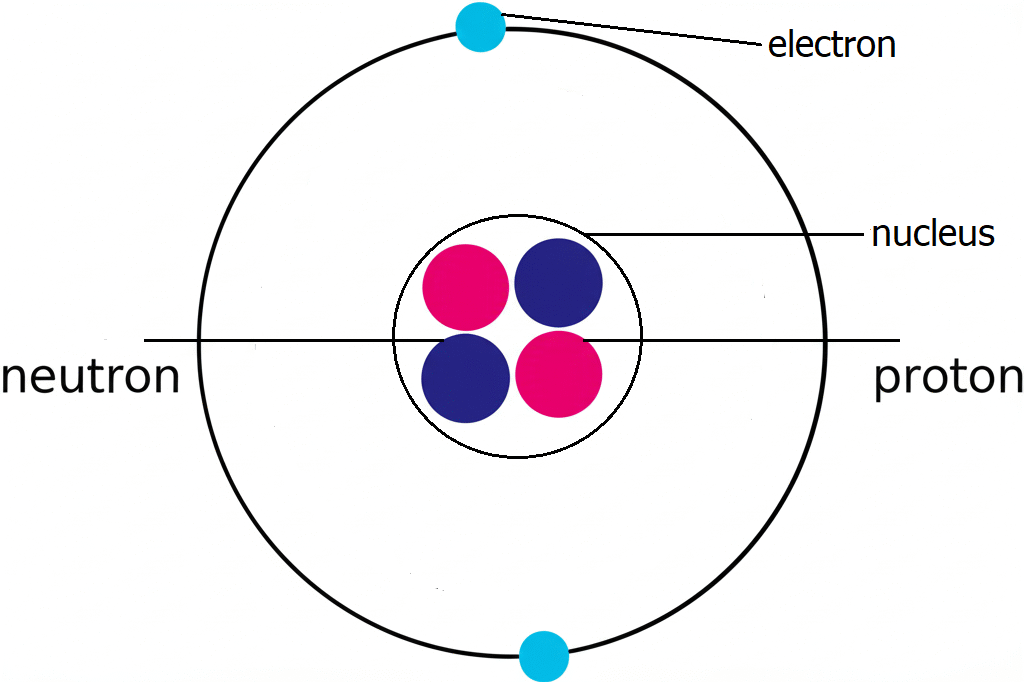
Atoms aren’t indivisible. In fact, each atom is made up of even smaller subatomic particles: The three main subatomic particles:
a. Protons
- Charge: Positive (+1)
- Location: Inside the nucleus
- Mass: Approximately 1 atomic mass unit (amu)
Protons define the atomic number, which determines the identity of an element. For example, carbon always has 6 protons, and oxygen always has 8.
b. Neutrons
- Charge: Neutral (0)
- Location: Inside the nucleus
- Mass: Slightly more than a proton, ~1 amu
Neutrons stabilize the nucleus by offsetting repulsive forces between positively charged protons.
c. Electrons
- Charge: Negative (−1)
- Location: Orbiting the nucleus in energy levels or shells
- Mass: ~1/1840 of a proton (negligible)
Electrons are responsible for chemical bonding and reactions. Though tiny, they determine how atoms interact.
Together, protons and neutrons form the nucleus (the dense center of the atom), while electrons move in regions around the nucleus called electron shells or orbitals.
So, what are atoms composed of? Protons, neutrons, and electrons.
Are Atoms Energy or Matter?
Atoms are forms of matter, not energy. Matter is anything that has mass and takes up space—and atoms fit that definition.
However, atoms can contain and transfer energy. Electrons in atoms can move to higher energy levels, and nuclear reactions (like in the sun) can convert mass into energy.
So while atoms are matter, they play a major role in how energy behaves in the physical world.
Are Atoms Indestructible?
No. In nuclear reactions, atoms can be split (fission) or fused (fusion), converting some mass into energy. These reactions power the sun and nuclear reactors.
But in everyday chemistry, atoms aren’t destroyed—they’re just rearranged. That’s why we say: mass is conserved in a chemical reaction.
Can We See Atoms?
Not directly with our eyes. But modern tools like the scanning tunneling microscope (STM) allow scientists to “see” individual atoms by mapping their surfaces.
Atomic Force Microscopy (AFM) and X-ray crystallography also help visualize atomic structures in materials and molecules.
These breakthroughs confirm much of what theory and experimentation have predicted for over a century.
What Are Atoms and Elements?
An element is a pure substance made entirely of one type of atom.
- Oxygen is an element made of oxygen atoms.
- Gold is an element made of gold atoms.
- Hydrogen gas (H₂) is made of hydrogen atoms bonded together.
All known elements are listed in the Periodic Table, and each element has its own unique type of atom.
So, the difference is:
- Atom = the building block.
- Element = a substance made from one kind of atom.
What Are Atoms Measured In?
Atoms are so small that we don’t measure them in everyday units like centimeters or grams.
- Atomic Mass: Measured in atomic mass units (amu). 1 amu = about the mass of one proton or neutron.
- Size: Atom diameters are measured in picometers (pm) or angstroms (Å). One atom is roughly 0.1 nanometers wide.
To compare: a single sheet of paper is about 1 million atoms thick.
What Are Atoms in Chemistry?
In chemistry, atoms are the basic units used to build molecules and compounds. They participate in chemical reactions by rearranging—not disappearing or forming from scratch.
That’s why in chemistry, we say:
Atoms are neither created nor destroyed in a chemical reaction—they’re simply rearranged.
This idea is key when we write chemical equations.
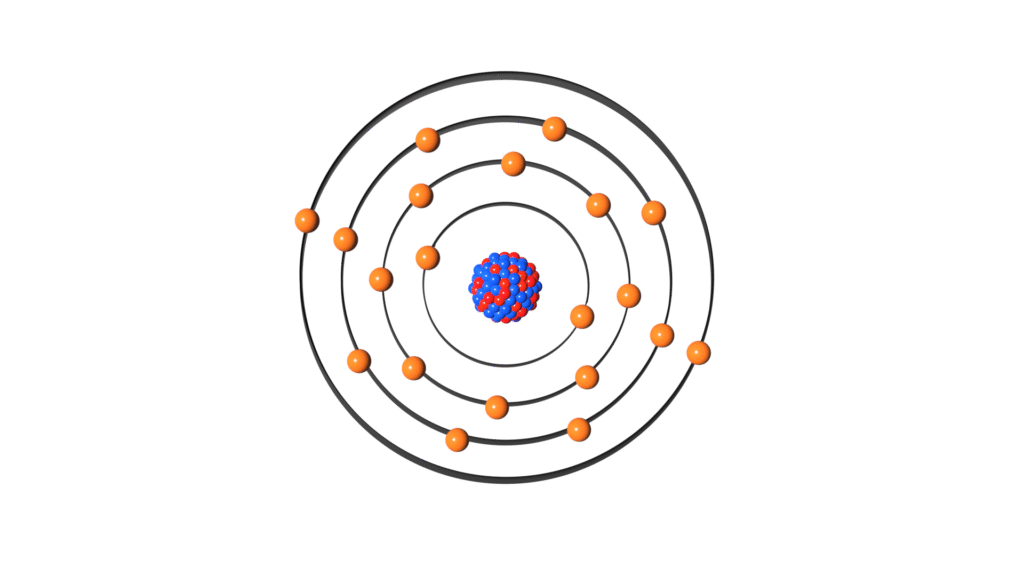
Atomic Structure: The Nucleus and the Electron Cloud
Atoms consist of a dense central nucleus surrounded by a cloud of electrons.
The Nucleus
Contains protons and neutrons. It’s extremely small but accounts for nearly all of an atom’s mass. If an atom were the size of a football stadium, the nucleus would be the size of a marble at the center.
The Electron Cloud
This is where electrons are likely to be found. It’s not a solid orbit but a probabilistic region described by quantum mechanics. Electrons are organized in energy levels or shells, with each shell holding a specific number of electrons:
- 1st shell: up to 2 electrons
- 2nd shell: up to 8 electrons
- 3rd shell: up to 18 electrons, and so on.
These arrangements are key to understanding how atoms bond. You can use 2ⁿ² (where n = 1, 2, 3, … represents the energy shell number) to determine the maximum number of electrons that can occupy a given energy shell.
Atomic Number and Mass Number
Every element on the periodic table is defined by two key numbers:
a. Atomic Number (Z)
This is the number of protons in an atom. It determines the element’s identity.
- Hydrogen has 1 proton → Atomic number = 1
- Carbon has 6 protons → Atomic number = 6
b. Mass Number (A)
This is the total number of protons plus neutrons.
- Carbon-12 → 6 protons + 6 neutrons = mass number of 12
- Oxygen-16 → 8 protons + 8 neutrons = mass number of 16
Electrons are not counted in mass number due to their tiny mass.
Types of Atoms: The 4 Main Types
There are many ways to classify atoms, but for beginners, here are the 4 main types of atoms based on their structure or behavior:
- Stable Atoms – These have balanced numbers of protons and neutrons and are not radioactive. Most natural elements are made of stable atoms.
- Isotopes – Atoms of the same element with different numbers of neutrons. For example, Carbon-12 and Carbon-14 are both carbon atoms.
- Ions – Atoms that have gained or lost electrons. If they lose an electron, they become positively charged (cation); if they gain one, they become negatively charged (anion).
- Radioactive Atoms – Unstable atoms that break down over time, releasing energy (radiation). These are found in nuclear materials and are used in medicine and archaeology.
Isotopes: Same Element, Different Mass
Isotopes are atoms of the same element with different numbers of neutrons.
For example:
- Carbon-12 has 6 protons and 6 neutrons.
- Carbon-14 has 6 protons and 8 neutrons.
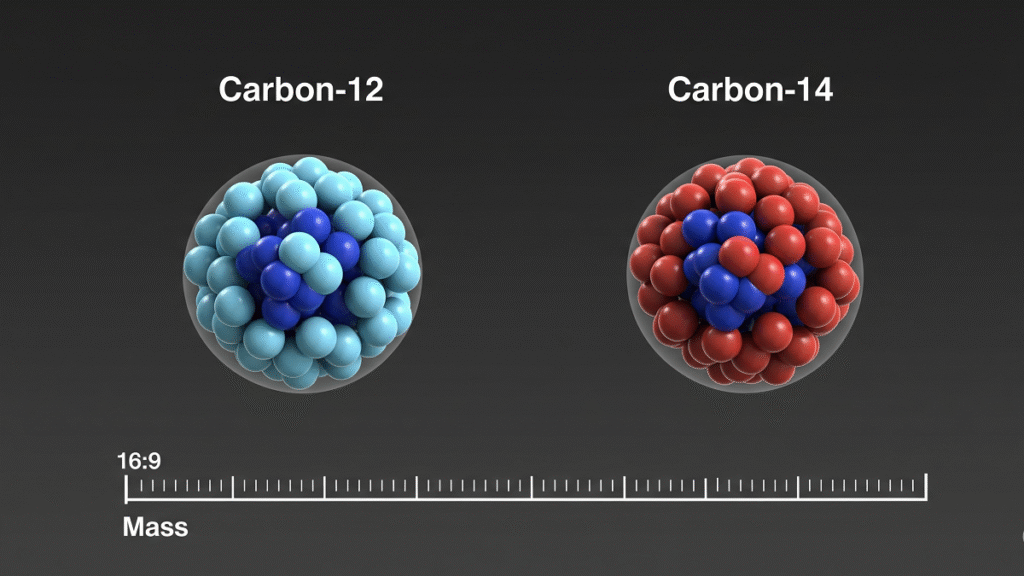
Both are carbon because they have 6 protons, but Carbon-14 is radioactive and used in radiocarbon dating.
Isotopes often behave the same chemically but differ in mass and nuclear stability.
Ions: Charged Atoms
An atom is neutral when it has the same number of protons and electrons. But it can lose or gain electrons to become an ion.
- Cation: Positively charged ion (lost electrons)
- Anion: Negatively charged ion (gained electrons)
Examples:
- Na⁺ is a sodium ion that has lost one electron.
- Cl⁻ is a chloride ion that has gained one electron.
Ions are essential in chemical reactions, electricity, and biological processes.
Atoms List: Examples of Common Atoms
Here’s a short list of common atoms (elements) and where they’re found:
These are all different types of atoms. Each has its own number of protons, which gives it its identity.
Atomic Models: How Our Understanding Evolved
a. Dalton’s Model (1803)
Atoms are solid, indivisible spheres. Useful but limited.
b. Thomson’s Plum Pudding Model (1897)
Discovered the electron. Atom is a positively charged “pudding” with negative “plums” (electrons).
c. Rutherford’s Nuclear Model (1911)
Gold foil experiment revealed atoms have a dense nucleus.
d. Bohr Model (1913)
Electrons orbit the nucleus in fixed energy levels. Still taught today for simplicity.
e. Quantum Mechanical Model (1920s–present)
The most accurate. Electrons exist in probabilistic orbitals, not fixed paths.
This model is based on quantum mechanics and explains phenomena like electron spin and orbital shapes.
The Periodic Table and Atomic Behavior
The Periodic Table arranges elements based on atomic number and recurring chemical properties.
Periods and Groups:
- Periods (rows): Indicate energy levels (shells)
- Groups (columns): Indicate similar chemical behavior
For example, Group 1 elements (alkali metals) are highly reactive, all having one electron in their outer shell. Group 18 elements (noble gases) are inert because their outer shells are full.
Atomic structure determines an element’s reactivity, melting point, electronegativity, and more.
What Are Atoms Held Together By?
Atoms are held together by forces—especially when they form compounds:
- Electromagnetic Force: Keeps electrons bound to the nucleus.
- Strong Nuclear Force: Holds protons and neutrons together in the nucleus.
- Chemical Bonds (like covalent or ionic bonds): Link atoms together in molecules and compounds.
For example, in a water molecule (H₂O), two hydrogen atoms and one oxygen atom are held together by covalent bonds, which share electrons.
Atoms rarely exist alone. They bond to form molecules and compounds. Chemical Bonds:
a. Ionic Bonds
Formed when electrons are transferred from one atom to another.
Example: Sodium (Na) donates an electron to chlorine (Cl) to form NaCl.
b. Covalent Bonds
Formed when atoms share electrons.
Example: Two hydrogen atoms share electrons with an oxygen atom to form water (H₂O).
c. Metallic Bonds
Electrons are shared in a “sea” among metal atoms, giving metals their conductivity and malleability.
The type of bond depends on the elements involved and their atomic structure.
What Are Atoms in a Chemical Equation?
In a chemical equation, atoms represent the reactants and products involved in a reaction.
Example:
2H₂ + O₂ → 2H₂O
This means:
- 4 hydrogen atoms and 2 oxygen atoms on the left (reactants)
- Rearranged into 2 water molecules on the right (products)
The atoms themselves don’t vanish—they’re just reorganized to form new substances.
Why Atoms Matter
Atoms are the foundation of chemistry. Without atoms, there would be no molecules, no water, no air, no life.
Chemists use their understanding of atoms to:
- Design new materials
- Develop medicine
- Produce cleaner fuels
- Understand environmental changes
Whether you’re studying how rust forms or how DNA works, it all starts with atoms.
Real-World Importance of Atoms
Understanding atoms helps explain everyday phenomena:
- Cooking: Heat causes atoms and molecules to rearrange.
- Medicine: Drug molecules interact with biological atoms.
- Electronics: Devices run on the movement of electrons (atoms’ outer particles).
- Environment: Greenhouse gases are molecules made of specific atoms—like carbon and oxygen.
Atoms may be tiny, but they govern every process, from rusting iron to breathing oxygen.
Surprising Facts About Atoms
- Most of an atom is empty space. If you removed the space in atoms, the entire human race could fit in a sugar cube.
- The atoms in your body are ancient—some originated in supernovae billions of years ago.
- There are about 7×10²⁷ atoms (7 octillion atoms) in your body. That’s a 7 followed by 27 zeros!
- Atoms are constantly moving. Even in solids, atoms vibrate in place.
- Atoms can bond in trillions of ways. This is why we have so many different substances in the world.
Atoms are not just physical units—they are cosmic stories, coded into matter.
Want More Chemistry Explained Simply?
👉 Subscribe to The Chemiverse Sage and get weekly insights that make science easy to understand.
How to Explain Atoms to Kids
What Are Atoms for Kids?
Atoms are like tiny building blocks that make up everything around us. Just like Legos can be put together to build a house, atoms can be joined to build water, people, air, and more.
How to Explain Atoms to a Child
Here’s how you might explain it to a young learner:
“Everything is made of atoms. You can’t see them because they’re super tiny, but they are everywhere. You are made of atoms, your clothes are made of atoms, and even the air you breathe is made of atoms!”
Use colorful visuals, simple analogies, and ball-and-stick models to make atoms fun and engaging.
FAQs About Atoms
An atom is the smallest unit of an element that still retains the properties of that element. It consists of a nucleus (with protons and neutrons) and electrons that orbit the nucleus.
Conclusion
From the early days of asking simple questions in science class to analyzing complex substances in a university lab, my journey through chemistry has always led back to one fundamental idea: atoms matter—literally and figuratively.
Through years of study, I’ve come to appreciate atoms not just as theoretical particles or symbols in a chemical equation, but as the silent architects of everything we know. Each drop of water, breath of air, flash of lightning, or burst of life is a dance of atoms—rearranging, bonding, and reacting in patterns that define our world.
And even now, with all I’ve learned—from atomic orbitals to quantum behavior—there’s still something deeply humbling about the fact that all of this complexity stems from such small, invisible building blocks.
So what are atoms?
They are not just a chapter in a textbook. They are the storytellers of nature, shaping matter and energy, and giving us the tools to understand everything from the beating of a heart to the glow of distant stars.
As a chemist, I don’t just study atoms—I see the world through them.
And I hope, now, you do too.
Let’s Talk Chemistry!
💬 Have a question about atoms or chemistry basics? Drop it in the comments below — I personally respond to every question.
Love Science That Makes Sense?
📚 Read more exciting Chemistry concepts—share it with a fellow science lover. You never know who might be curious about the world around them.
Test What You’ve Learned
🧠 Ready for a quick quiz? Click here to try a 5-question mini-quiz on atoms and see how much you remember!



